Publication
The in vivo study of the device with 15 dialysis patients has been published in the ‘Hemodialysis International’ journal. Please find the link to the article below:
Dialysis
Dialysis, often referred to as the ‘artificial kidney’, is a lifeline for individuals facing renal failure. In this condition, the kidneys struggle to effectively filter waste and excess fluids from the body, posing a significant threat to overall health.
Imagine the kidneys as natural filters for our bloodstream, removing harmful substances and maintaining the body’s fluid balance. When these vital organs falter, dialysis steps in as a crucial support system. It mimics some of the essential functions of the kidneys, ensuring that patients continue to live.
Beyond its role as a ‘life-saver,’ dialysis brings a multitude of benefits. It boosts energy levels, manages electrolyte balance, and alleviates other symptoms associated with kidney failure. At Sedign Solutions, we understand the profound impact of dialysis on patients’ quality of life and are committed to advancing this vital modality.
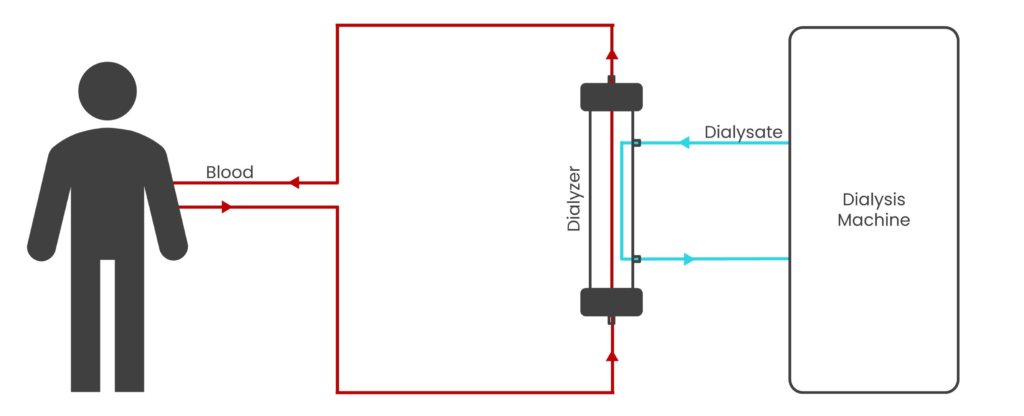
Fig 1: Schematic diagram representing hemodialysis
Hemodialysis, a crucial medical procedure, involves circulating a patient’s blood through a specialized machine that acts as an artificial kidney. This machine effectively filters out waste products and excess fluids from the blood before returning it to the body as shown in Fig 1. Hemodialysis sessions, typically performed two to three times a week, last for three to fours hours each. The purification employs a semi-permeable membrane housed within a filter (known as a dialyzer) to remove solutes from the blood. The principle is predominantly based on diffusion, where solutes in the blood move at high velocity, collide with the membrane, and pass through its pores from a region of high concentration to low concentration. In addition to solutes, excess fluid is removed from the blood by a process known as ultrafiltration. It relies on convection of plasma water across the membrane due to an applied trans-membrane pressure (TMP). Thus, hemodialysis is a pressure-driven membrane separation process employing both diffusion and convection.
Small-solute clearance (measured as Kt/Vurea) is considered as a priority to achieve adequate dialysis and is the most frequent measure of the delivered dose of dialysis. K, in the term Kt/V, is the dialyzer clearance, t is the duration of dialysis and V is the distribution volume of urea in the body. To ensure effective dialysis, the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommend a target single pool Kt/V (spKt/Vurea) of 1.4 per hemodialysis session for patients treated thrice weekly, with a minimum delivered spKt/Vurea of 1.21,2.

Status Quo
In the pursuit of achieving the target Kt/V efficiently, renal care providers need to achieve a delicate balance. While the urea distribution volume (V) for a given patient is relatively fixed, providers have some degree of control over the two other critical variables: time (t) and dialyzer clearance (K). Increasing the time of dialysis, whether by increasing the duration or frequency of sessions, can enhance solute removal, thus elevating Kt/V. However, this approach comes at a cost, as it necessitates additional resources, making it an economically challenging choice. Alternatively, providers can optimize dialyzer clearance (K), a parameter denoting the hypothetical volume of blood cleared of specific solutes per minute during dialysis, typically measured in mL/min. Maximizing K offers a viable avenue to boost Kt/V safely and economically, improving the effectiveness of hemodialysis without incurring substantial resource burdens.
Fig 2 elucidates the intricate relationship between clearance (K) and key dialysis parameters. To increase clearance (K) and enhance hemodialysis efficiency, several strategies can be employed. First, elevating the blood flow rate (Qb), which represents the rate at which blood is drawn from the patient and circulated through the machine, results in higher clearance. Second approach involves selecting a dialyzer with a higher KoA value, achieved by opting for a larger dialyzer with an increased surface area (A) or by augmenting the permeability coefficient (Ko) of the dialyzer, leading to a higher diffusive flux (φ) given by equation 1.1: 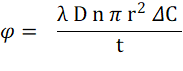
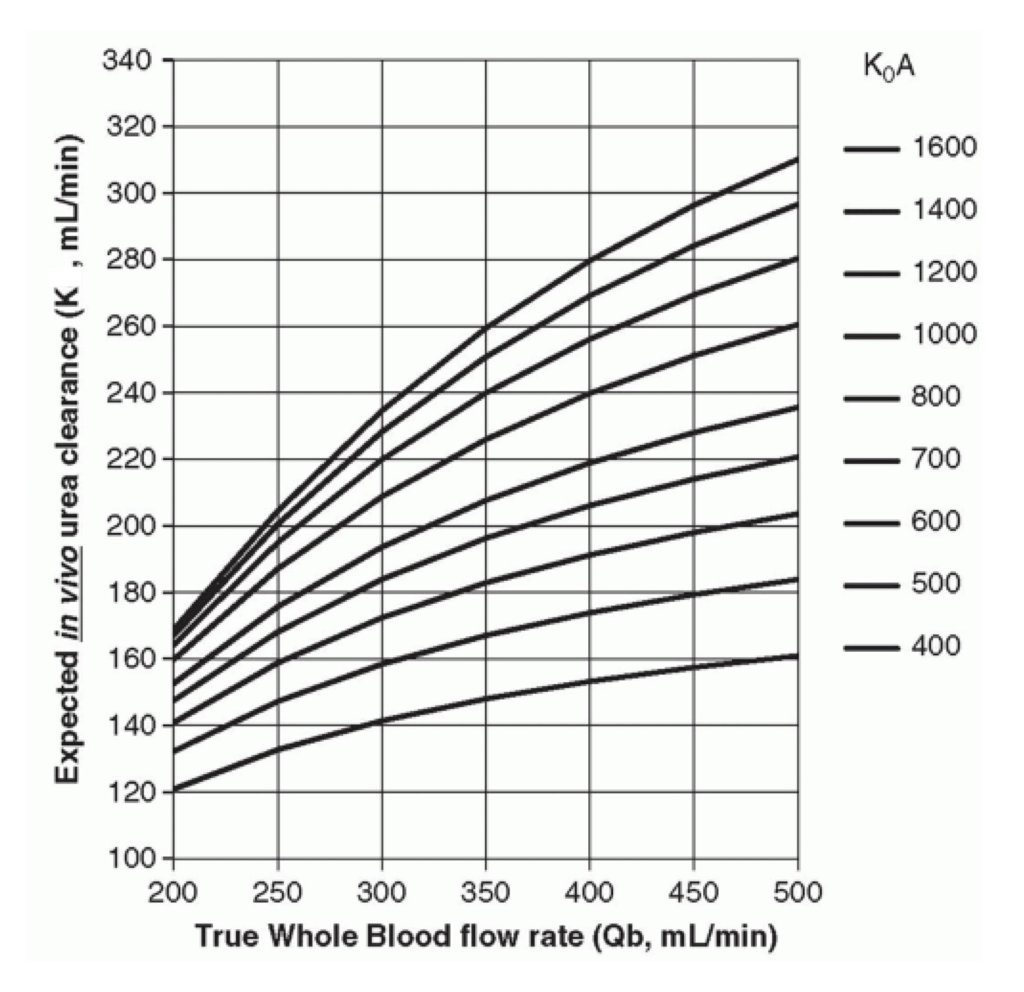
Fig 2: Parameters determining clearance [9]
where λ is the solute partition coefficient, D is the solute diffusivity, n is the number of pores per unit area, r is the pore radius, ΔC is the transmembrane concentration gradient, and t is the membrane wall thickness3.
The permeability coefficient (Ko) and flux (φ) can be enhanced through various methods, including reducing membrane thickness (t), increasing pore number (n) or radius (r), and optimizing dialysate flow paths using techniques like inter-fiber spacer yarns or fiber undulation (crimping). Commercial dialyzers leverage these strategies to enhance dialysis efficiency. They are categorized as: ‘low-flux’ with small-pored membranes for low molecular weight solute removal, and ‘high-flux’ with larger pores to additionally eliminate larger solutes.
Our project’s inception aimed to identify novel, cost-effective approaches for enhancing the performance of conventional dialysis equipment.
Long-Standing Oversight
In any filtration process utilizing a sieve, like a membrane, efficiency tends to decline due to clogging of the sieve over time. In hemodialysis, blood constituents such as erythrocytes, platelets, plasma proteins and solutes accumulate on the membrane surface, gradually obstructing its pores. As the dialysis session progresses, fouling and concentration polarization (CP) layers form on the blood side of the membrane4. Partial pore clogging decreases pore radius (r), while complete clogging reduces the total pore count (n) for solute transport, leading to a flux (φ) reduction as per equation 1.1. These alterations significantly impact the dialyzer membrane’s diffusive and convective properties.
In high-flux dialyzers, CP visibly affects ultrafiltration as shown in Fig 3. The larger membrane pores facilitate the rapid transfer of plasma water, leading to a rapid rise in protein concentration. As a result, a CP layer forms on the blood-side of the membrane, increasing the oncotic pressure and impeding further ultrafiltration6. ‘Reverse filtration’ may occur if the oncotic pressure exceeds the TMP, undermining the purpose of using a high-flux dialyzer.
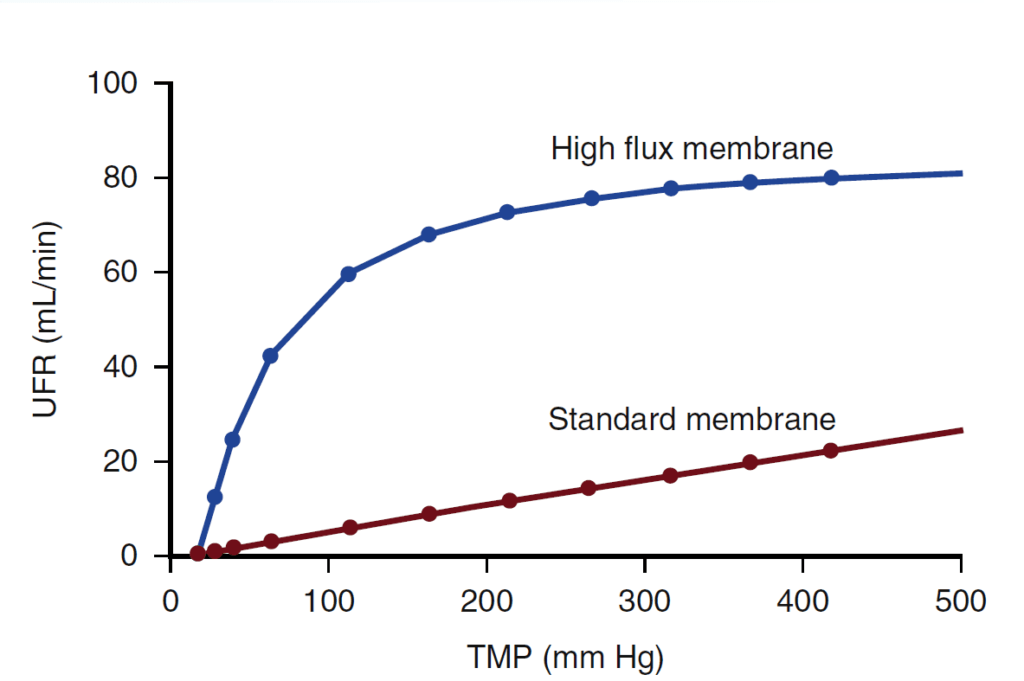
Fig 3: Impact of CP on high flux dialysis [6]
Our clinical investigation which involved 15 patients on maintenance hemodialysis, found that fouling and CP visibly affected the rate of removal of solutes (known as clearance) as shown in Fig 4. The clearance was being monitored using Online Clearance Monitoring (OCM) of the Fresenius 4008S dialysis machines. A significant reduction in small-solute clearance by up to 27.8% was observed during the four-hour sessions.
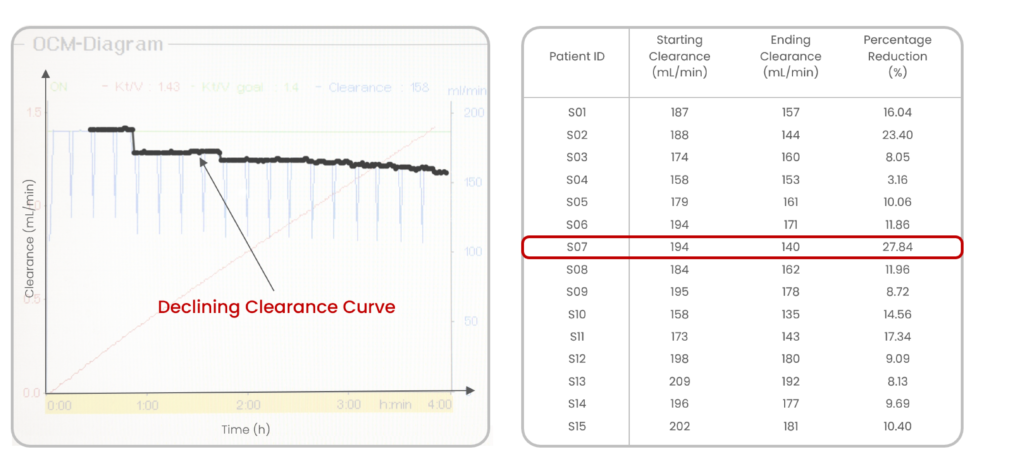
Fig 4: Intra-dialytic reduction of clearance captured by OCM (our results)
The findings parallel those of Morty et al.7, reporting a modest reduction of less than 20% in urea clearance over the course of a three-hour dialysis session. More importantly, their study emphasized a much more substantial reduction in clearance for larger molecules, with dextran experiencing a staggering 90% reduction (molecular weights: dextran-25,000 Da; urea-60 Da). This reduction in clearance occurs progressively, with the bulk of it transpiring between the 40 and 120-minute mark of the dialysis session.
Intra-dialytic membrane clogging remains an evident but unsolved challenge in dialysis. This phenomenon results in a notable decrease in both the flux of solutes and ultrafiltration rates, consequently diminishing the overall effectiveness of a dialysis session. The area under the OCM plot, reflecting the product of clearance (K) and time (t), directly correlates with session adequacy (Kt/V). A substantial decline in clearance (K) values during the session corresponds to a proportional decrease in dialysis adequacy (Kt/V). Notably, as this clogging phenomenon occurs during the session itself, structurally or chemically modifying a dialyzer during its manufacturing proves insufficient in mitigating it. Thus, a pressing need exists for a mechanism that effectively combats fouling and concentration polarization during dialysis.
A Game-changing Solution
Introducing Diaraise, an innovative dialysis equipment accessory designed to combat intra-dialytic membrane clogging. This non-invasive device seamlessly attaches to the dialyzer without direct contact with process fluids and is compatible with all existing dialysis equipment.
In our clinical investigations, Diaraise exhibited remarkable efficacy, with a mean percentage reduction in clearance values of only 4.41% compared to 12.69% in regular sessions (p<0.001). Diaraise effectively dislodges fouling and CP layers, preventing the reduction of pore radius (r) and pore number (n) described in Equation 1.1. Consequently, a larger membrane surface area (A) is available for solute transport, leading to an increased diffusive flux (φ) and improvements in session adequacy.
Safety of patients and dialysis equipment is of utmost importance. Diaraise was effectively demonstrated to be safe. No significant differences were observed in blood parameters, and no alarms were triggered on the dialysis machine during its application.
This technology is versatile and can be applied across various dialysis modalities, including low-flux, high-flux, hemodiafiltration, and SLED. Its adaptability makes it a valuable tool for addressing membrane clogging and enhancing dialysis efficiency across different renal-care settings. Particularly in high-flux dialysis, Diaraise has the potential to improve ultrafiltration rates, prevent back-filtration, and facilitate more effective convective solute transport.
Achieving optimal clearance of solutes during a dialysis session remains a major challenge. The incorporation of Diaraise with the dialysis process has demonstrated promising results in mitigating membrane clogging. By enhancing the clearance of solutes, Diaraise can help achieve better quality of life of patients, higher operational efficiency and improved profitability. The implementation of this technology holds the potential to revolutionize dialysis by addressing a critical unmet need in the field of renal care.
References
- National Kidney Foundation. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. 2015;66(5):884-930
- Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis (HEMO study). N Engl J Med. 2002;347(25):2010–9.
- Lysaght, M. J. Hemodialysis membranes in transition. Nephrology and Dialysis Updated 1988, 61, 1-17.
- Huang Z, Gao D, Letteri JJ, Clark WR. Blood-membrane interactions during dialysis. Semin Dial. 2009 Nov-Dec;22(6):623-8
- Clark W. R., Gao D., Neri M., & Ronco C. Solute transport in hemodialysis: advances and limitations of current membrane technology. Expanded Hemodialysis 2017, 191, 84-99.
- Gilbert S. J, Weiner D.E., Ing. “Hemodialysis” National Kidney Foundation’s Primer on Kidney Diseases, Edition 6, Elsevier Saunders, pp.508-519
- Morti, S. M., & Zydney, A. L. (1998). Protein-membrane interactions during hemodialysis: effects on solute transport. ASAIO Journal (American Society for Artificial Internal Organs: 1992), 44(4), 319-326.
- Header Image: collection of images from SEM analysis of dialyzer membranes from our study, increasing in magnification from left to right: x22, x450, x10000.
- Fig 2: Daugirdas, J. T., Blake, P. G., & Ing, T. S. (2012). Handbook of Dialysis. Lippincott Williams & Wilkins.
