The project commenced in 2018 as part of a ‘Social Innovation Immersion Program’ which was initiated by Biotechnology Industry Research Assistance Council (BIRAC) under the aegis of the Department of Biotechnology, Government of India. This initiative aimed to enhance the quality of life for the elderly through innovative solutions.
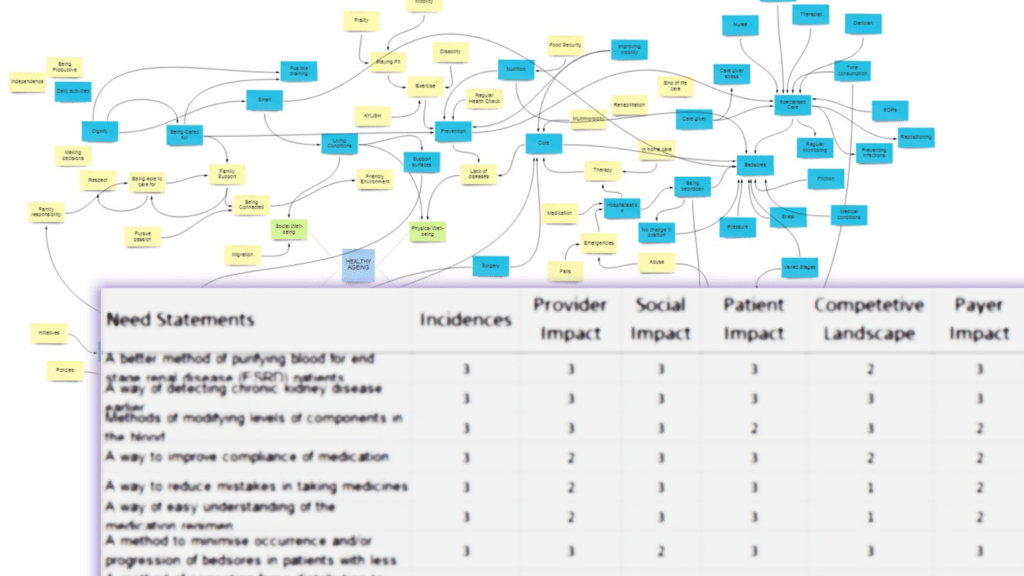
During six months of clinical immersions, several hospitals and old age homes were visited, resulting in over 500 key observations. With the valuable guidance from BIRAC experts and clinicians, ‘end-stage renal care’ was chosen as the area of interest. This led to a focused clinical immersion in four dialysis centers over the next six months.

After interacting with patients, their relatives, nephrologists, dialysis technicians, dialysis center administrators, dieticians, and industry experts, 147 key observations were filtered down to 28 precise need statements in the realm of renal care, encompassing both hemodialysis and peritoneal dialysis.

Extensive study of the dialysis process, comprehensive literature searches, market analysis, and feedback from experts collectively culminated in the selection of the need statement: ‘Improve clearance of solutes in hemodialysis.’ It prompted in-depth study of the theories governing membrane separation processes, hemodialysis procedures, machine operations, and dialyzer membrane properties.
Furthermore, dialysis data collection on adequacy served to validate the issue of ‘Dialysis Inadequacy.’ Subsequent investigations unearthed concentration polarization (CP) as a pivotal contributor to this inadequacy problem. It also became evident that concentration polarization significantly impacted the clearance of middle and large molecules in high-flux dialyzers.
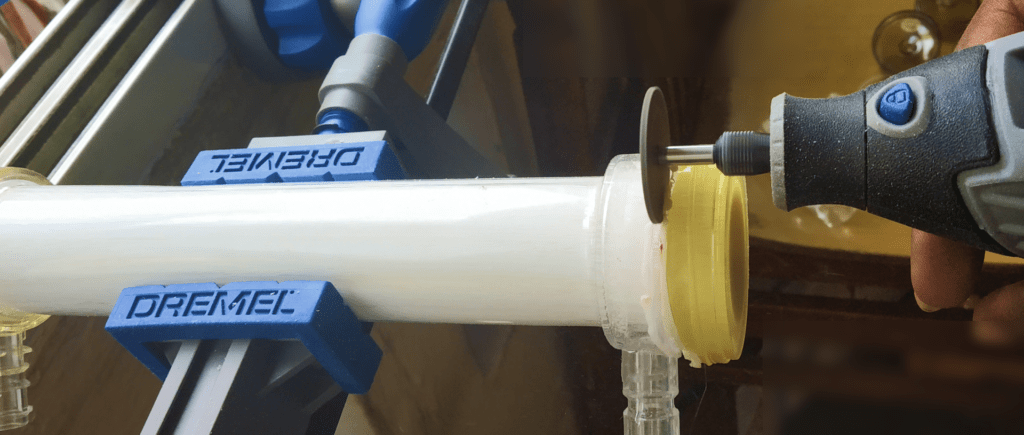
Following a meticulous examination of the issue, various methods for mitigating and preventing concentration polarization were evaluated. Among these mechanisms, one emerged as the most promising after rigorous bench testing and literature research. During the ideation phase, critical factors such as safety, manufacturability, and seamless integration with existing dialysis equipment were all taken into account.
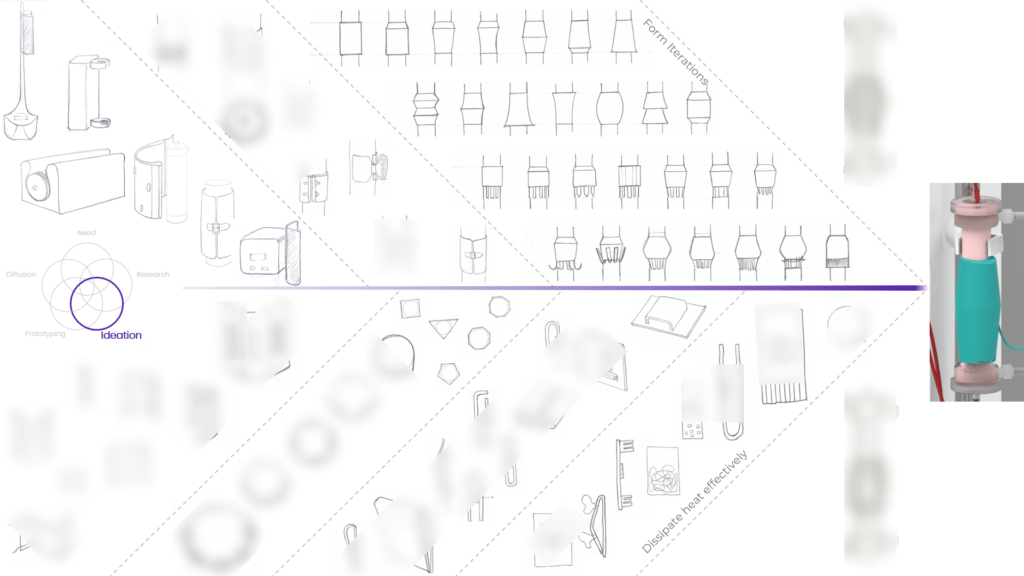
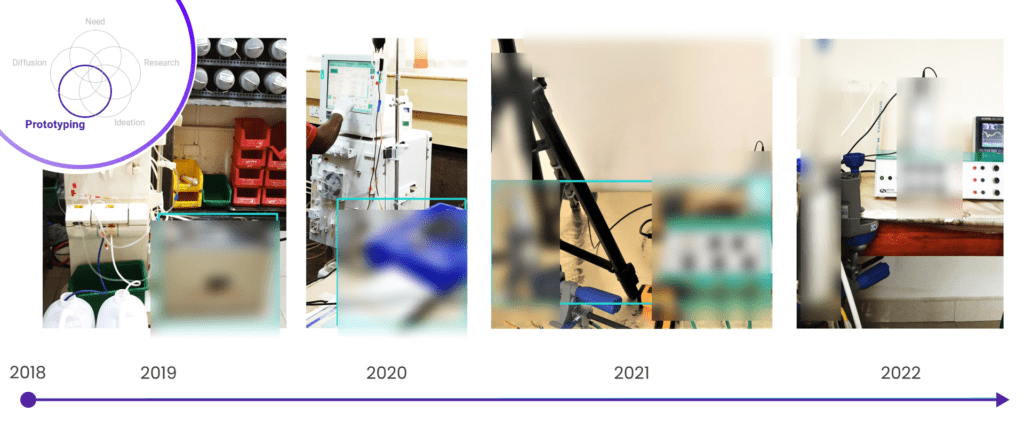
As we ventured into uncharted territory, we faced many challenges. We had our fair share of setbacks, with prototypes that didn’t work and tests that didn’t go as planned. However, we eventually created a solution that has proven to be both safe and effective in a real-world clinical setting!
We extend our heartfelt gratitude to the numerous organizations and individuals who have supported us on this journey thus far.

As we move forward, we are excited about the prospect of forming new partnerships and collaborations to take this solution to the global audience.
